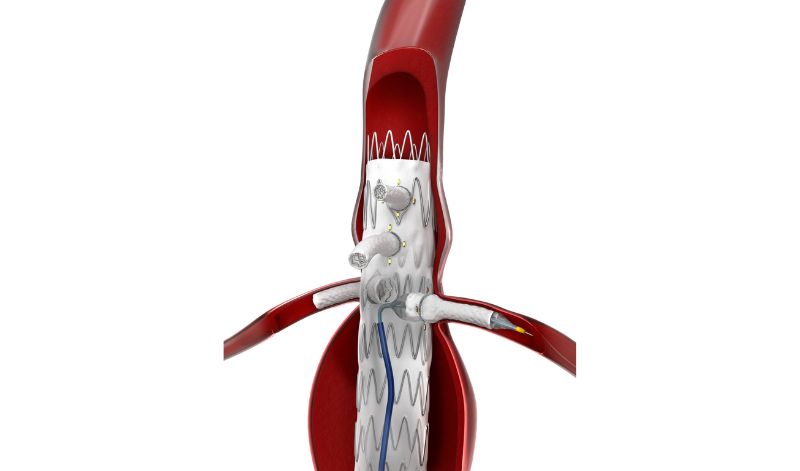
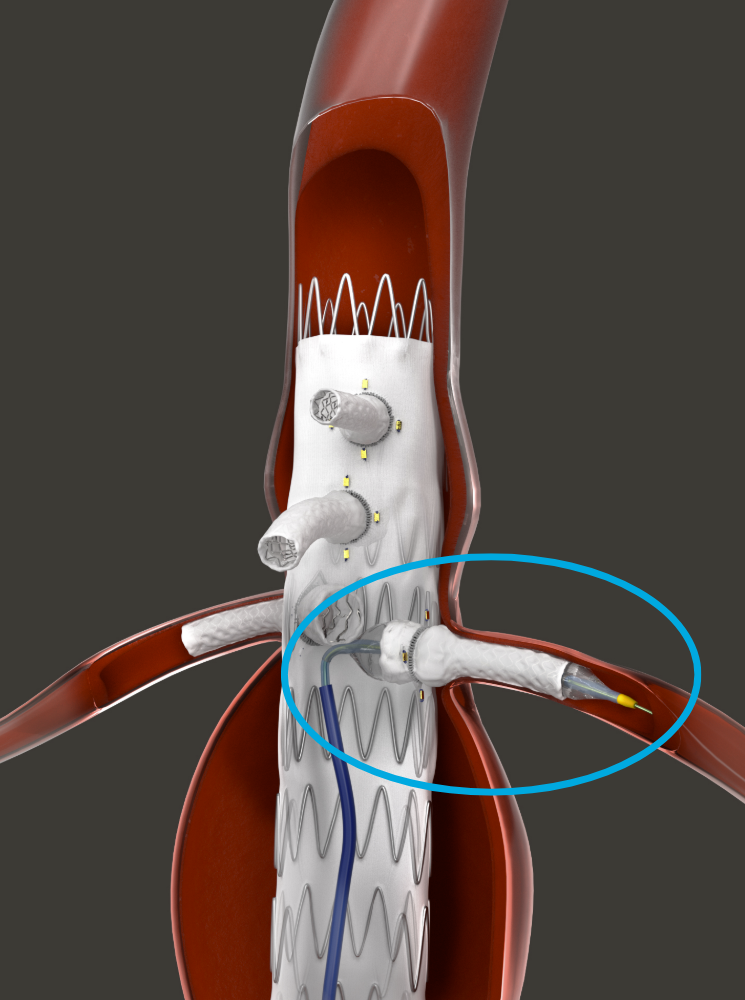
HECHINGEN, GERMANY - The first patient to undergo fenestrated endovascular aortic repair (FEVAR) with the Bentley (Bentley InnoMed, Hechingen, Germany) BeGraft peripheral balloon expandable covered stent in the BeGraft FEVAR Study is being treated today.
The patient was referred for FEVAR with a 6 cm juxta-renal abdominal aortic aneurysm, explained the coordinating investigator and study lead, Professor Eric Verhoeven, professor of vascular surgery at the General Hospital Nuremberg, Paracelsus Medical University, Nuremberg, Germany. “This patient has an absolute indication due to the diameter and we cannot use standard EVAR to seal below the renal arteries because there is no sealing zone available.
A further two patients are planned in the next week, added Professor Verhoeven, pleased to announce the launch of the open-label BeGraft FEVAR study.
The study aims to investigate the safety and performance of the BeGraft peripheral Stent Graft System as a bridging stent in FEVAR for complex aortic aneurysms. The study, which is approved by the German competent authority BfArM, is being run in collaboration with Bentley and the Foundation for Cardiovascular Research and Education (FCRE).
Participating centres in the study include Nuremberg, Munster, Munich, Regensburg, Aachen, Stuttgart, and Giessen, all in Germany.
A total of 100 patients will be included to generate data on approximately 250-300 BeGrafts in fenestrations. Recruitment is deemed to take 12 months, with 24 months follow-up.
Main outcomes will be technical success (successful introduction and deployment), bridging stent patency at 12 months, as well as the absence of procedure-related complications and bridging stent-related endoleaks at 12 months. Overall clinical outcomes including 30-day mortality, and health-related quality of life will also be assessed alongside adverse events.
There are numerous covered stents on the market, including the BeGraft, all used off-label as bridging stents in FEVAR. However, there is a lack of consensus around what to use and there is no gold standard or solid evidence to support their use.
“Bentley is the first company to try to obtain an on-label approval for the BeGraft stent in FEVAR, and shift from off-label to on-label use,” remarked Professor Verhoeven.
In the meantime, FEVAR is a well-established, minimally-invasive option for people with abdominal aortic aneurysms who are not eligible for a traditional endovascular aneurysm repair (EVAR).These patients, who represent around 10% of all patients, usually have an aneurysm that is too close to the renal arteries, and until recently, they would require open surgery or no treatment at all.
Open surgery is more invasive, and mortality is higher. In 2016, Professor Verhoeven published results of a study that showed mortality in patients who undergo FEVAR at his centre was 0.7%[1]. There is also a reduced duration of recovery in hospital with FEVAR compared to open surgery.
“For me, it’s clear that the BeGraft has an advantage as it goes into a 6F guiding sheath rather than a 7F sheath. The slightly lower profile is important, as a 6F guiding sheath tracks difficult anatomy better than a 7F. Also, the BeGraft comes in many lengths, and in short intervals, like a 27/28mm length, which is often ideal for more difficult renal arteries,” added Professor Verhoeven.
Bentley is also conducting a prospective, single arm, multi-centre clinical study to obtain an indication for the use of the BeGraft Peripheral Plus as a bridging stent in branched endovascular aortic repair (BEVAR) for the treatment of complex thoraco-abdominal aortic aneurysms. This trial began in September 2020 and aims to carry out 100 BEVAR procedures with an average of 2,5 bridging stents per procedure.
Initial results of the BeGraft in FEVAR trial should be available around mid-2023.
ClinicalTrials.gov Identifier: NCT03987035
[1] Verhoeven EL, Katsargyris A, Oikonomou K, et al. Fenestrated endovascular aortic aneurysm repair as a first line treatment option to treat short necked, juxtarenal and suprarenal aneurysms. Eur J Vasc Endovasc Surg 2016;51(6): 536-42.
Contact: Kerstin Stotz, Communication & Event Coordinator, k.stotz@bentley.global, +49747198499559

Professor Eric Verhoeven and his team at General Hospital Nuremberg, Paracelsus Medical University, Nuremberg, Germany after the first BeGraft implantation for the FEVAR clinical trial study